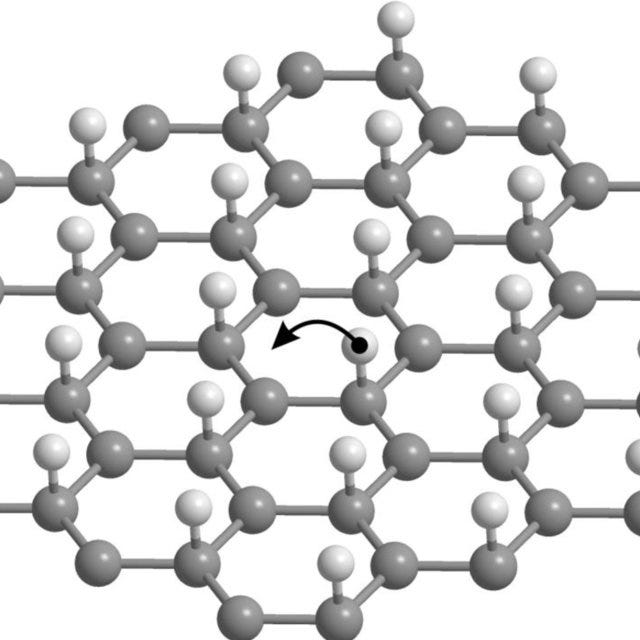
Graphene derivatives
Graphane, fluorographene, graphene oxide, graphone, graphyne and graphdiyne
Graphane
The addition of hydrogen to graphene causes deformation of the original flat monoatomic graphite layer, because all carbon atoms in the resulting lattice change their hybridisation from the flat sp2 to the tetrahedral sp3. This modification transforms the conducting graphene into the dielectric graphane. The theoretical possibility of such a structure has been proven by J. O. Sofo; the first graphane samples were experimentally obtained by teams from Chernogolovka and Manchester in 2009.
As graphene converts into fully hydrogenated graphane via intermediate partially hydrogenated structures, the electronic properties of the material undergo gradual changes. It is therefore expected that controlled incomplete hydrogenation of graphene may provide materials with the desired properties. Similarly, hydrogenation of predefined domains of a graphene sheet to graphane could be the way to "cut out" graphene fragments of desired shape, although such controlled hydrogenation would be a non-trivial task of own importance.
Fluorographene
A stoichiometric derivative of graphene with a fluorine atom attached to each carbon is reported. Raman, optical, structural, micromechanical, and transport studies show that the material is qualitatively different from the known graphene-based nonstoichiometric derivatives. Fluorographene is a high-quality insulator (resistivity >1012 Ω) with an optical gap of 3 eV. It inherits the mechanical strength of graphene, exhibiting a Young’s modulus of 100 N m−1 and sustaining strains of 15%. Fluorographene is inert and stable up to 400 °C even in air, similar to Teflon.
The material was first described in 2010 by Robinson et al using graphene grown on copper foil exposed to xenon difluoride at 30 °C. The group of Nair et al. started from cleaved graphene crystals on a gold grid also exposed to xenon difluoride, at 70 °C. Also in 2010 Withers et al. described exfoliation of fluorinated graphite (monolayer, 24% fluorination) and Cheng et al. reported reversible graphene fluorination. The stoichiometric fluorographene (CF) was also prepared by chemical exfoliation of graphite fluoride by Zboril et al. Zboril et al. also showed that the graphene fluoride can be transformed into graphene via graphene iodide, a spontaneously decomposing intermediate
Graphene oxide
Graphite oxide, formerly called graphitic oxide or graphitic acid, is a compound of carbon, oxygen, and hydrogen in variable ratios, obtained by treating graphite with strong oxidizers and acids for resolving of extra metals. The maximally oxidized bulk product is a yellow solid with C:O ratio between 2.1 and 2.9, that retains the layer structure of graphite but with a much larger and irregular spacing.
The bulk material spontaneously disperses in basic solutions or can be dispersed by sonication in polar solvents to yield monomolecular sheets, known as graphene oxide by analogy to graphene, the single-layer form of graphite. Graphene oxide sheets have been used to prepare strong paper-like materials, membranes, thin films, and composite materials. Initially, graphene oxide attracted substantial interest as a possible intermediate for the manufacture of graphene. The graphene obtained by reduction of graphene oxide still has many chemical and structural defects which is a problem for some applications but an advantage for some others.
Graphone
Graphone is a half-hydrogenated graphene. The structure of graphone is illustrated as trigonal adsorption of hydrogen atoms on graphene at first. However, we found the trigonal adsorption is unstable. We present an illustration in detail to explain how a trigonal adsorption geometry evolves into a rectangular adsorption geometry. We check the change of magnetism during the evolution of geometry by evaluating the spin polarization of the intermediate geometries. We prove and clarify that the rectangular adsorption of hydrogen atoms on graphene is the most stable geometry of graphone and graphone is actually antiferromagnetic.
Graphyne
Graphyne is a little known sibling of graphene. Simulations have shown that graphyne's conduction electrons travel extremely fast – as they do in graphene – but in only one direction. That property could help researchers design faster transistors and other electronic components that process one-way current.
Graphyne is distinct in being composed of sp and sp2 carbon atoms, which contrasts with graphene containing only sp2 carbon.
Graphynes and graphdiynes are generic names for families of 2D carbon allotropes with honeycomb structure, where acetylenic groups connect the hexagons of graphene, with the coexistence of sp and sp2 hybridized carbon atoms. The main differences between graphynes and graphdiynes are the number of acetylenic groups (one for graphynes and two for graphdiynes).
The coexistence of sp and sp2 carbons in graphyne gives rise to unique physical properties, such as high conductivity and large carrier mobility. This makes it a promising candidate for several application areas (which the authors discuss in great detail in their review): electrocatalysis; organic reaction catalysis; energy storage devices; photocatalysts and solar cells; water purification; and sensors.
Graphdiyne
Graphdiyne (GDY), a new two-dimensional (2D) carbon allotrope, has been receiving increased attention. Its unique sp–sp2 carbon atoms, uniform pores, and highly π-conjugated structure provide promising potential in practical applications, such as gas separation, catalysis, water remediation, humidity sensor, and energy-related fields. In the recent years, considerable efforts have been expended toward the development of well-defined GDY. However, GDY materials still face numerous challenges, including the need for a more thorough understanding of the growth mechanism, strategies for synthesizing one- or few-layer single-crystalline GDY films, characterization of basic physicochemical properties, and achievement of promising applications. This review aims at providing a comprehensive update on the synthesis of GDY and GDY-based materials, as well as their properties, including structural, electronic, mechanical, and spectral properties, and their applications in nanotechnology.
Different from other carbon allotropes, graphdiyne (GDY) features topological sp- and sp2-hybridized carbon atoms. With the unique structural advances, a charming electronic behavior has been presented by GDY, which has shown great improvement for various energy-related fields. Inspired by this, it is time to summarize the structure-induced property enhancement. Firstly, different stacking configurations in GDY can induce specific electronic properties, bringing promising energy application. Secondly, uniform pores provide enough sites for anchoring atoms and nanoparticles, enriching the diversity of materials. Thirdly, the sufficient alkynyl groups offer active sites for doping and grafting, providing possibilities for the precise design at the molecular level. Lastly, we propose some perspectives for future trends on GDY. Through this review, we hope to provide a guideline for rational design on GDY-based materials and reveal the structure-performance relationship between functionalized GDY and energy conversion/storage.
Don't hesitate to subscribe if you haven't already done so.
Regards and take care.
Born in 2012, Scixel is a project devoted to the improvement of the scientific comunication through the creation of graphical products: pictures, animations, graphs, posters, etc. Scixel consists of scientists with a deep knowledge in digital graphics but also with a long experience in giving talks, preparing posters and papers and other daily situations of scientific work.
We have focused our work into universities and research institutes all over the world: TuDelft (The Netherlands), NIMS (Japan), Basel University (Switzerland), Universidad Autónoma de Madrid, CNB or ICFO (Spain), to name a few.
Web: https://scixel.es/
If you are a company or an individual who would like to place your advertising in my newsletter you can contact me (email) and let me know your request of type of ad and number of newsletters you would like to place it. I will send you a budget as soon as possible.